In industries such as pharmaceuticals, biotechnology, and electronics manufacturing, maintaining a clean and sterile environment is crucial. This guide will provide an overview of the different levels of clean room classification and the specific requirements for each level. Whether you’re new to the industry or just need a refresher, this guide will help you understand the importance of cleanroom classification and how to maintain a sterile environment.
What is a clean room and why is it important?
A cleanroom is a controlled environment that is designed to minimize the introduction, generation, and retention of airborne particles and other contaminants. It is important because in industries such as pharmaceuticals, biotechnology, and electronics manufacturing, even the smallest particle or contaminant can have a significant impact on the quality and safety of the products being produced. Cleanrooms help ensure that products are manufactured in a sterile and controlled environment, reducing the risk of contamination and ensuring product quality and safety.
Understanding clean room classifications.
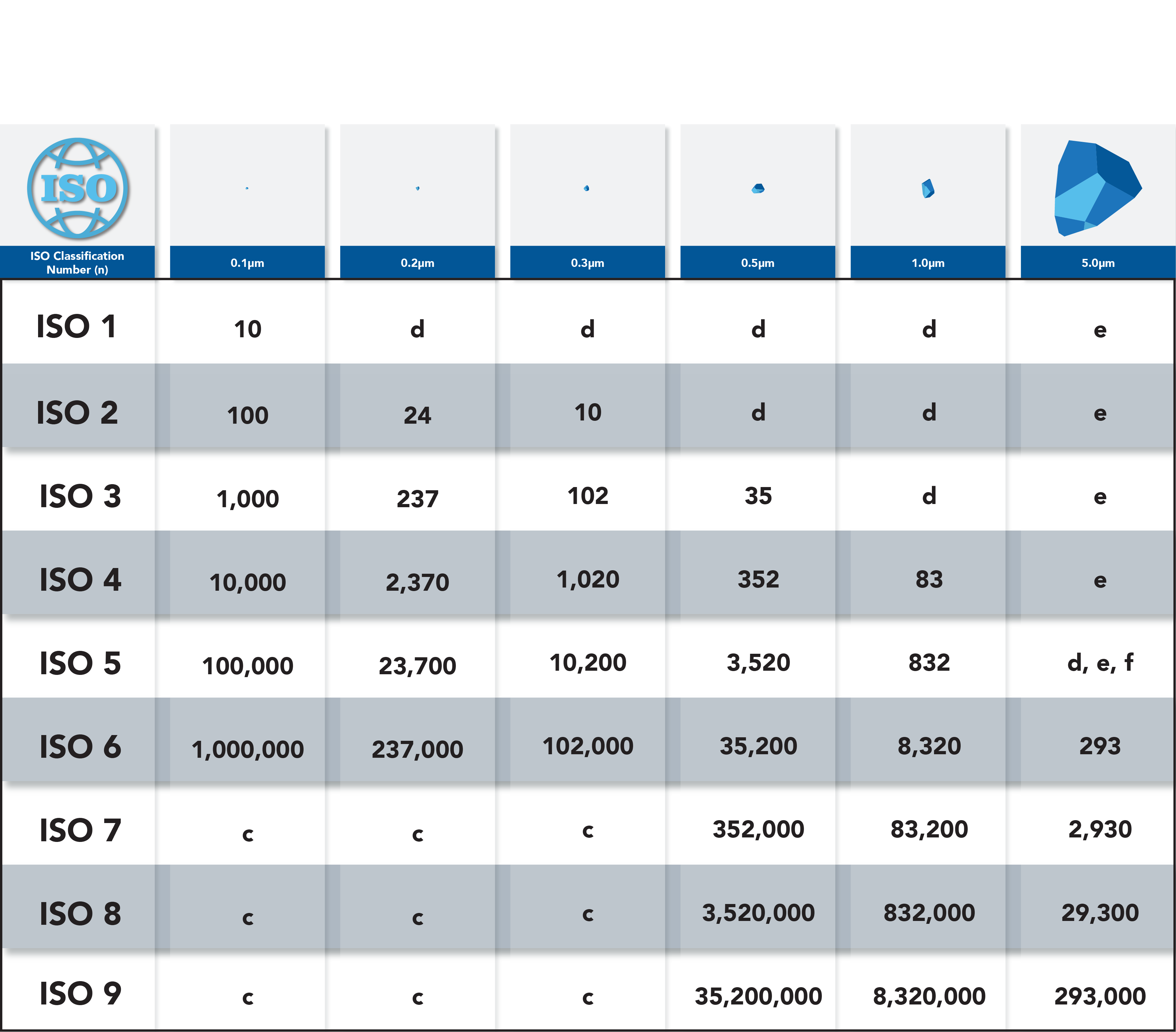
Cleanrooms are classified based on the number and size of particles allowed per cubic meter of air. The classification system ranges from ISO Class 1 (the cleanest) to ISO Class 9 (the least clean). Each classification has specific requirements for air filtration, air changes, and cleanliness levels. Understanding the different classifications is important for ensuring that the cleanroom is appropriate for the intended use and that products are manufactured in a controlled and sterile environment.
Requirements for each clean room classification.
Each cleanroom classification has specific requirements for air filtration, air changes, and cleanliness levels. ISO Class 1 cleanrooms, for example, require the highest level of cleanliness with no more than 10 particles per cubic meter of air larger than 0.1 microns. ISO Class 9 cleanrooms, on the other hand, allow up to 35,200 particles per cubic meter of air larger than 0.5 microns. It’s important to understand the requirements for each classification to ensure that the cleanroom is appropriate for the intended use and that products are manufactured in a controlled and sterile environment.
Maintaining a clean room environment.
Maintaining a cleanroom environment requires strict adherence to cleaning protocols and procedures. This includes regular cleaning and disinfection of surfaces, equipment, and tools, as well as proper gowning and hygiene practices for personnel entering the cleanroom. It’s important to have a comprehensive cleaning plan in place and to regularly monitor and test the cleanliness levels to ensure that the cleanroom is meeting the required standards. Any deviations from the cleaning plan or cleanliness levels should be immediately addressed to prevent contamination and maintain the integrity of the cleanroom environment.
Choosing the right cleaning products and equipment.
When it comes to cleaning a cleanroom, choosing the right cleaning products and equipment is crucial. Not all cleaning products and equipment are suitable for use in a cleanroom environment, as they may introduce contaminants or particles that could compromise the cleanliness levels. It’s important to use products and equipment that are specifically designed for use in cleanrooms and that meet the required standards for cleanliness. This may include specialized mops, wipes, disinfectants, and vacuum cleaners that are designed to minimize the release of particles and contaminants. Regular maintenance and calibration of cleaning equipment is also important to ensure that they are functioning properly and effectively.
continue reading